Healthy mitochondria are vital to normal cellular activities. Mitochondrial dysfunction drives the pathogenesis of a wide variety of medical disorders, including acute conditions and chronic degenerative diseases. Distinct aspects of mitochondrial function, for example, bioenergetics, dynamics, and cellular signaling are well described and impairments in these activities likely contribute to disease pathogenesis. Due to the complexity of the disorders, a single approach may not lead to effective therapeutics. Mitobridge utilizes an integrated platform of assays and models to systematically evaluate disease-related changes in mitochondrial function and delivers innovative medicines.
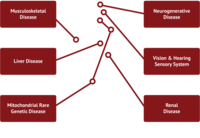
Given the myriad of roles that mitochondria play in cellular homeostasis, it is no surprise that defects in mitochondrial function can lead to a broad spectrum of diseases. These functional defects are most apparent in tissues that have a high energy demand such as skeletal muscle, kidney, liver, heart and central nervous system (CNS).
Mitochondrial genetic diseases are caused by defects in either nuclear or mitochondrial genes. This group of disorders, which includes Leigh’s Syndrome, Leber's hereditary optic neuropathy, and MELAS can be associated with a variety of symptoms and could clinically benefit from treatments that improve mitochondrial function.
Diseases associated with mitochondrial dysfunction are common yet diverse and can manifest as kidney disease, liver disease, and heart disease, hearing loss, muscular dystrophies and myopathies. Mitochondrial dysfunction is also associated with aging, and has been shown to play a role in neurodegenerative diseases. The etiology of certain “emerging” indications such as Chronic Fatigue Syndrome and Sarcopenia is not well understood, but recent studies have indicated that targeting mitochondria to enhance their function may hold promise for attenuating those conditions.
Mitochondrial-directed therapies may have the ability to address multiple molecular abnormalities simultaneously and may prove to be more efficacious than compounds that target an isolated protein. Mitobridge has the opportunity to develop drug candidates that could be therapeutically beneficial in a multitude of disease indications. Our strategy is to quickly establish proof of concept in rare diseases with high unmet medical need and then expand into the more prevalent diseases.

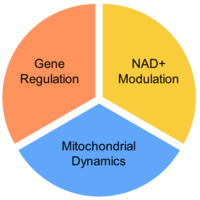
Gene regulation – One of the mechanisms cells use to manage protein requirements is gene regulation. Exercise, a type of cellular stress, induces gene regulation as a response to increased energy demand. Mitobridge scientists have identified molecules that can regulate the gene sets controlled by exercise – these compounds are called “exercise mimetics”.
NAD+ enhancement –NAD+ plays many important roles within cells, including serving as an oxidizing agent in oxidative phosphorylation which generates ATP from ADP. Increasing cellular concentrations of NAD+ will enhance the oxidative capacity within mitochondria, thereby increasing nutrient oxidation and boost energy supply, which is a primary role of mitochondria.
Mitochondrial dynamics – Mitochondrial health depends on several complex processes that help cells function under normal and stress conditions. Mitochondrial dysfunction can lead to mitochondrial clearance (mitophagy) or cell death (apoptosis). Mitochondria are dynamic organelles, able to change their morphology by undergoing fusion or fission. Many diseases stress mitochondria, which can disrupt the normal dynamics processes.
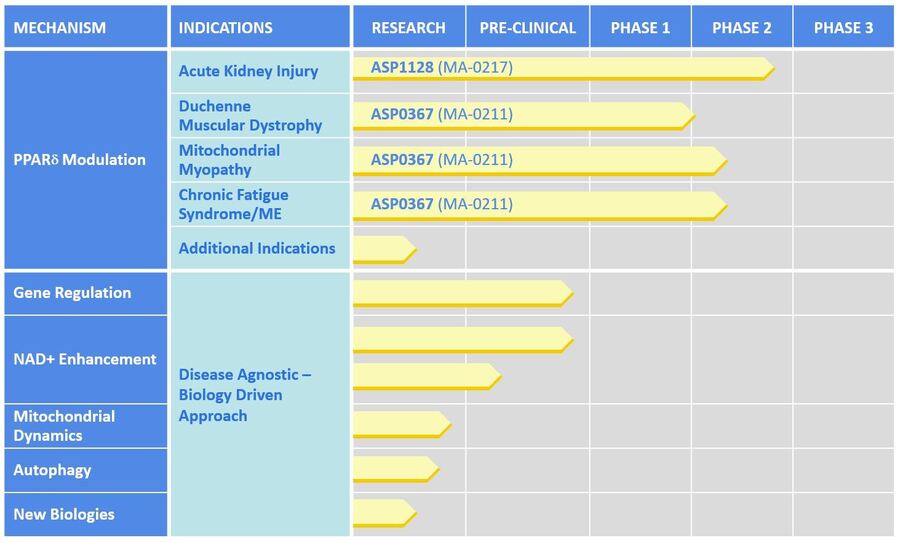
Our scientists are applying expertise in mitochondrial biology to discover and develop a pipeline of therapeutics to improve mitochondrial function for patients, including in kidney and muscle diseases. Our lead drug development programs are focused on modulation of PPARδ, a key regulator involved in improving muscle endurance and increasing mitochondrial fatty acid utilization.
ASP1128 is a potentially first-in-class approach to treating acute kidney injury (AKI). ASP1128 is believed to have protective effects on kidney cells that are under cellular stress for patients at increased risk of AKI following coronary artery bypass and/or valve (CABG/V) surgery by promoting fatty acid oxidation in the mitochondria. A proof of concept Phase 2 study with ASP1128 is ongoing.
ASP0367 offers a mitochondrial-directed approach for the treatment of Duchenne Muscular Dystrophy (DMD) by increasing fatty acid oxidation and mitochondrial biogenesis in muscle cells. ASP0367 has successfully completed Phase 1 clinical trials and is scheduled to enter a safety and efficacy study in DMD patients in early 2020.